Abstract
Purpose:
Endothelial vulnerability is a novel concept to understand complications after allogeneic stem cell transplantation (alloSCT). The concept is based on genetic polymorphisms in the recipients' thrombomodulin gene (THBD) and endothelial related serum markers prior to conditioning therapy. These parameters predict outcome of alloSCT. CD40 ligand (CD40L) is a key orchestrator of immune responses. Its expression on endothelial cells led us to study single nucleotide polymorphisms (SNPs) in the recipients' CD40L gene on outcome after alloSCT.
Patients and methods:
Three CD40L gene SNPs (rs3092920, rs30929252, rs3092936) were correlated with outcome endpoints in 342 alloSCT recipients. The SNPs had at least 10% minor allele frequency and tagged additional 21 polymorphisms encompassing 30 kb region of gene locus. The chosen three polymorphisms are in linkage disequilibrium with CD40LG haplotype (TGGC) of rs975379, rs3092952, rs3092933 and rs3092929 polymorphisms that was associated with increased circulating soluble CD40 ligand in inflammatory disorders.
Overall survival (OS), non-relapse mortality (NRM), acute GVHD-related mortality, and transplant-associated microangiopathy (TAM) were analyzed. The genotype relevant to outcome was subsequently tested in an independent cohort (n=364) and merged into a genetic risk score with established THBD polymorphisms (rs1962, rs1042579 and rs1042580 (Rachakonda, et al., JCO 2014)). Validation of this risk score was attempted in the independent cohort. Finally, the score was evaluated in 375 independent, non-randomized patients taking statin-based endothelial prophylaxis.
Results:
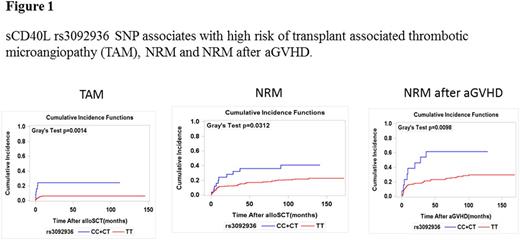
While rs3092920 and rs30929252 had no impact on any endpoint, rs3092936 CC/CT genotype was associated with an increased risk of TAM (HR 4.83, p=0.001, 95%CI, 1.89-12.37), NRM (HR 2.24, p=0.019, 95%CI, 1.15-4.40) and acute GVHD-related mortality (HR 3.02, p=0.005, 95%CI, 1.39-6.56) (Figure 1). The combined genetic high risk score (CD40L and THBD) predicted OS and NRM in uni- and multivariable analyses (Multivariable analysis, training cohort, OS: HR 1.50, 95% CI 1.07-2.01, p=0.018; NRM: HR 2.52, 95% CI 1.54-4.13, p=0.0003; validation cohort, OS: HR 1.51, 95% CI 1.05-2.17, p=0.025; NRM: HR 2.17, 95% CI 1.25-3.78, p=0.006). In order to confirm and validate the prognostic impact of the genetic risk score on NRM in patients without statin therapy, the cause-specific Cox model was fitted to the validation cohort with an offset equal to the effect of the genetic risk score in the training cohort. The effect of THBD+CD40L score in the validation cohort was not significantly different from the effect in the training cohort.
The predictive impact of the genetic risk score was completely lost in patients taking statins.
Conclusion:
The rs3092936C allele in the recipients' CD40L gene independently associates with NRM and risk of TMA. A combined genetic score based on THBD and CD40L polymorphisms strongly predicted outcome, but only in the absence of a statin based endothelial protection. Our results support the hypothesis that endothelial vulnerability can be defined prior to allogeneic stem cell transplantation by genetic markers in the recipient's genome. The normalization of mortality risk in patients treated with statins suggests a way of overcoming the negative impact of high risk genotypes and warrants further clinical validation.
Luft: Alexion: Consultancy; Neovii: Research Funding. Dreger: AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Riemser: Consultancy, Research Funding; Riemser: Consultancy, Research Funding; Riemser: Consultancy, Research Funding; Jansen: Consultancy; Gilead: Consultancy, Speakers Bureau; Riemser: Consultancy, Research Funding; Riemser: Consultancy, Research Funding; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Riemser: Consultancy, Research Funding; Riemser: Consultancy, Research Funding; Riemser: Consultancy, Research Funding; Jansen: Consultancy; Riemser: Consultancy, Research Funding; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Riemser: Consultancy, Research Funding; Jansen: Consultancy; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Riemser: Consultancy, Research Funding; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Jansen: Consultancy; Jansen: Consultancy; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Jansen: Consultancy; Gilead: Consultancy, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Jansen: Consultancy; Riemser: Consultancy, Research Funding; Gilead: Consultancy, Speakers Bureau; Jansen: Consultancy; Jansen: Consultancy; medac: Other: Travel grants; medac: Other: Travel grants; medac: Other; medac: Other; medac: Other; Gilead: Consultancy, Speakers Bureau; Jansen: Consultancy; Gilead: Consultancy, Speakers Bureau; Jansen: Consultancy; Gilead: Consultancy, Speakers Bureau; medac: Other: Travel grants; Jansen: Consultancy; Gilead: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; medac: Other: Travel grants; Gilead: Consultancy, Speakers Bureau; medac: Other: Travel grants; medac: Other: Travel grants; medac: Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal